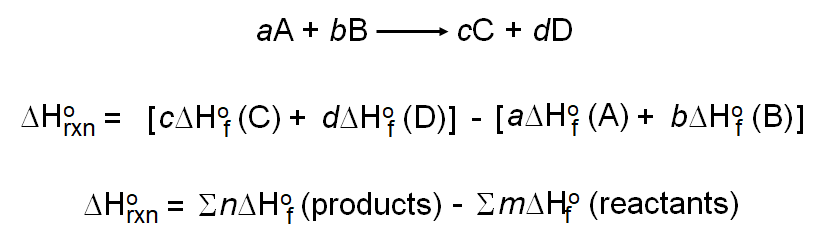Question Video: Calculating the Enthalpy Change for the Reaction between Phenol and Diatomic Hydrogen Using Standard Enthalpies of Combustion | Nagwa

If the standard internal energy change for the reaction OF2(g) + H2O(g)⟶ O2(g) + 2HF(g) , at 298 K is x kJ . [Given standard enthalpies of formation in kJ mol^-1 are

How would you calculate the standard enthalpy change for the following reaction at 25 °C: H2O (g) + C (graphite)(s) --> H2 (g) + CO (g)? | Socratic
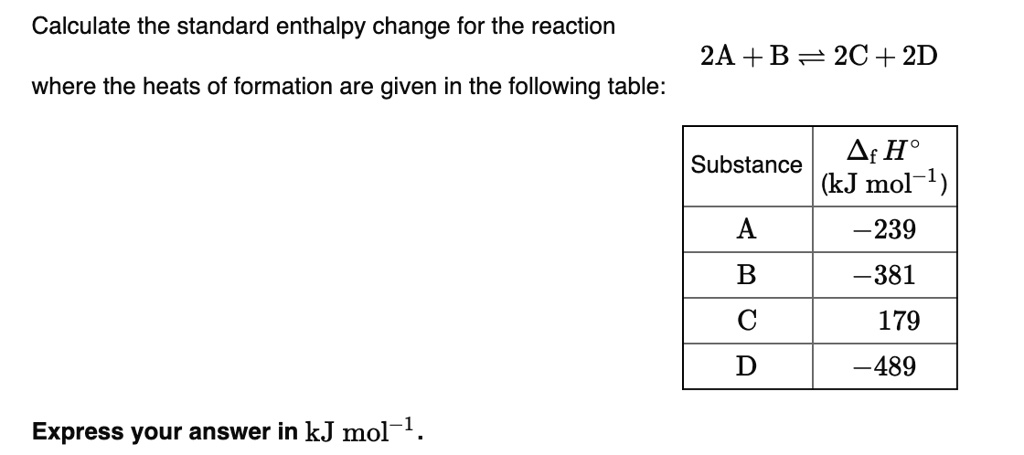
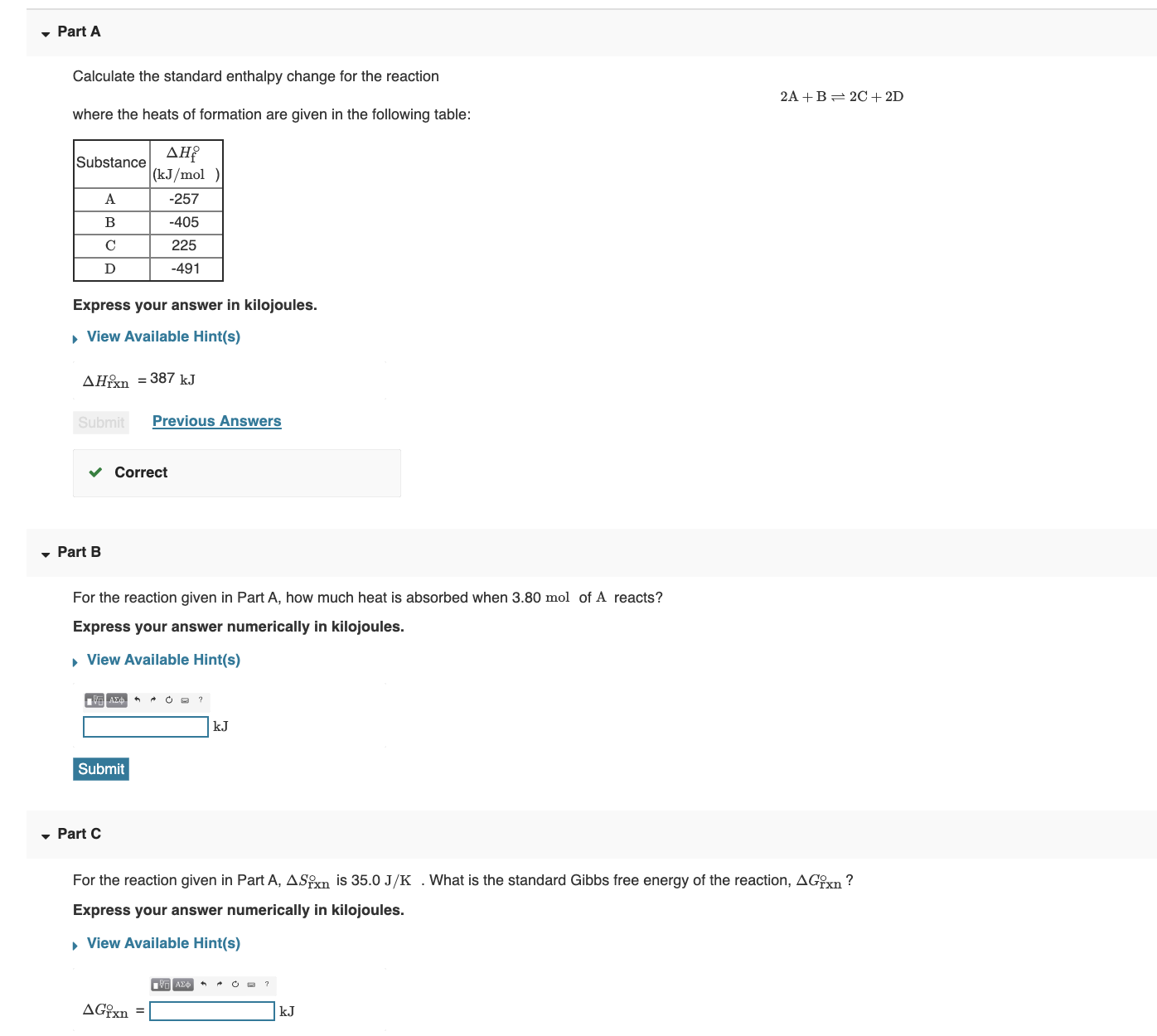
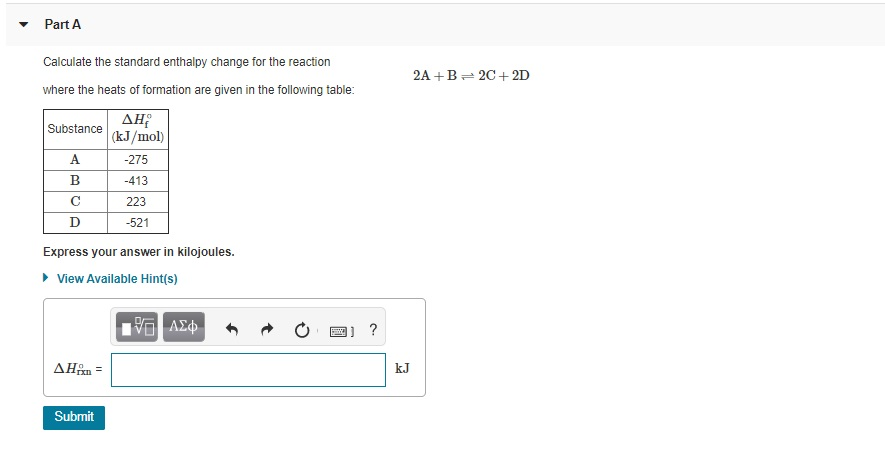
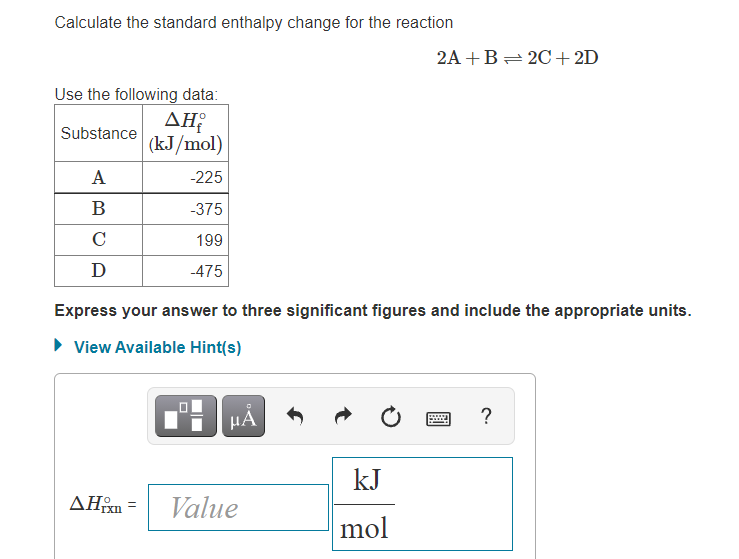
SOLVED: Calculate the standard enthalpy change for the reaction 2A +B = 2C + 2D where the heats of formation are given in the following table: Af Ho Substance (kJ mol-1) A
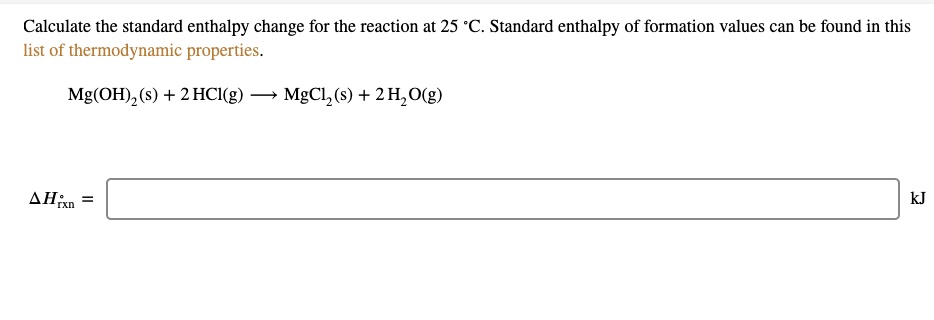
SOLVED: Calculate the standard enthalpy change for the reaction at 25 'C. Standard enthalpy of formation values can be found in this list of thermodynamic properties. Mg(OH) (s) + 2 HCl(g) MgCl,(s) +
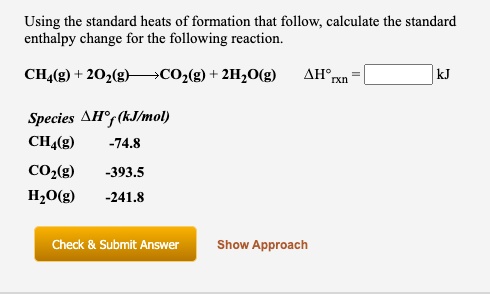
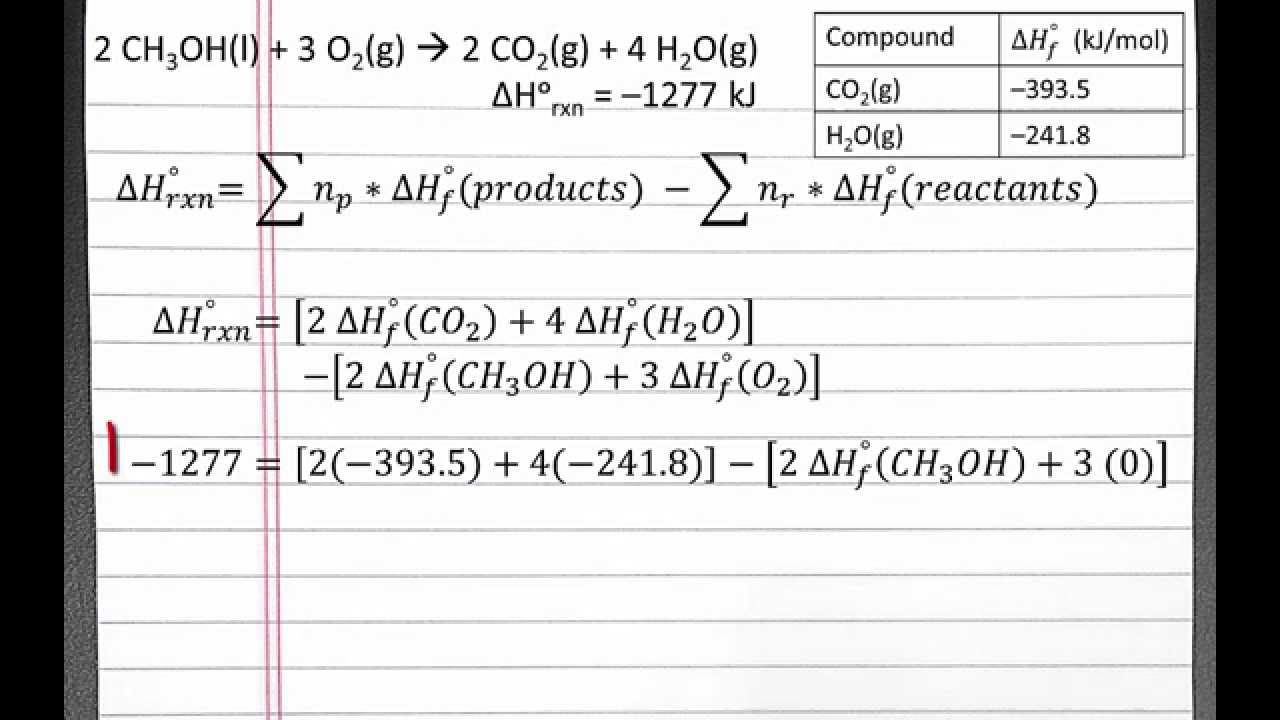
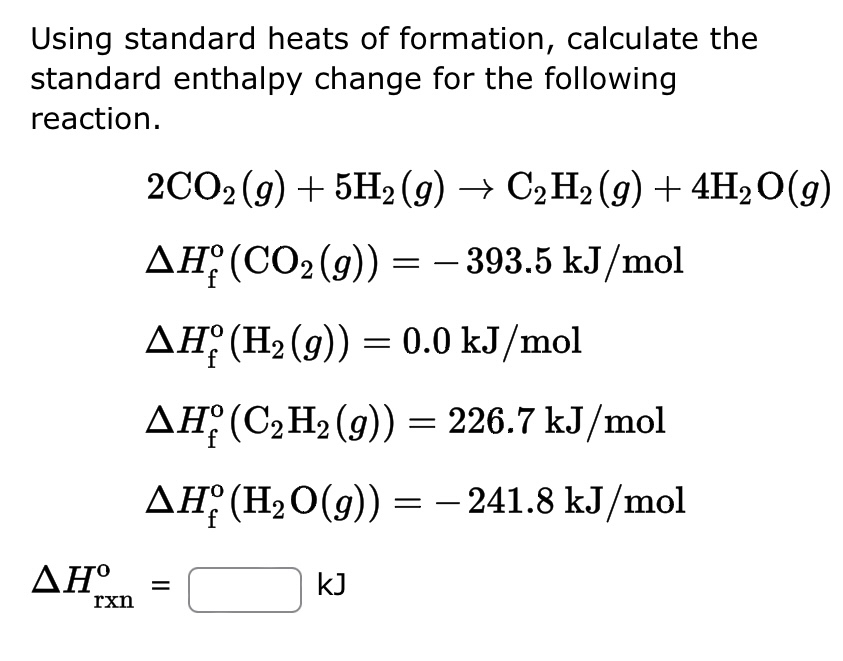
SOLVED: Using the standard heats of formation that follow; calculate the standard enthalpy change for the following reaction. CHy(g) 202(g) COz(g) 2H,O(g) AHCrxn Species AH (kJlmol) CHy(g) -74.8 COz(g) 393.5 HzO(g) 241.8